Introduction of laboratory
■ Name of laboratory
Department of Integrated Molecular Imaging (Cooperative Lab)
■ Research contents
【1】Early prediction of efficacy of anticancer treatment by molecular imaging
【2】 Diagnostic imaging of atherosclerotic lesions (vulnerable plaques)
【3】Development of new imaging agent using thymidine phosphorylase (TP) as a target
Department of Integrated Molecular Imaging
(Cooperative Lab)
― Main themes ―
- Research to explore molecules that are most suitable for the evaluation of the functional changes in molecules and cells, that is, the target molecules in the analysis of pathological conditions and imaging, from the viewpoint of the evaluation of pathological conditions and treatment efficacy and the establishment of treatment strategy
- Research on development of molecular probes that can clarify the functions of enzymes and receptors related to various pathological conditions at the molecular level
- Research on the application of the latest pathological analysis and imaging techniques using tracers, such as molecular imaging, to diagnosis and treatment
- Research on the development of a new imaging technique for evaluating the treatment efficacy and precisely understanding in vivo pharmacokinetics by combining the latest pathological analysis techniques using tracers, such as molecular imaging, with advanced drug-discovery techniques.
- Research on the evaluation of treatment efficacy and prediction of side effects on the basis of the analysis of in vivo pharmacokinetics of drugs by radiotracer method
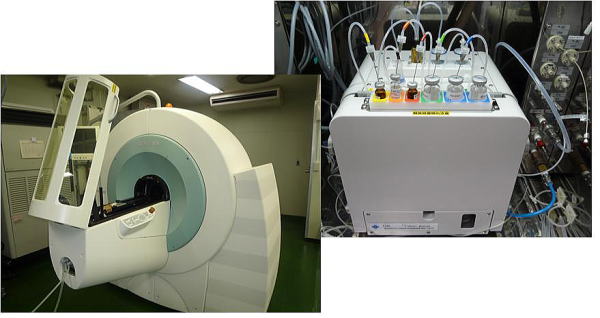
(Figure) Imaging apparatus for animals installed in the Central Institute of Isotope Science (PET/SPECT/CT) and probe (18FDG) synthesizer installed in Hokkaido University Hospital
Introduction of research
【1】Early prediction of efficacy of anticancer treatment by molecular imaging
(1) Evaluation of efficacy of molecular targeted therapy
(gefitinib) by molecular imaging for assessing tumor growth potential
The use of a molecular targeted drug called gefitinib (trade name, Iressa),
which selectively inhibits the epidermal growth factor receptor (EGFR),
was first approved in Japan. With its clinical efficacy clarified, gefitinib
has made significant contributions to the treatment of cancer. However,
it takes a long time to determine the efficacy of molecular targeted anticancer
drugs because they need to be continuously administered to patients at
a certain or higher dose for them to exert their anticancer effect. For
the assessment of the anticancer effect, conventional techniques such as
computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography
require a long time to detect a decrease in cancer size; thus, it is considered
difficult to determine early treatment efficacy using these techniques.
The anticancer effect can be observed at the molecular and cellular levels
(e.g., metabolism and apoptosis) before morphological changes become apparent.
Therefore, molecular imaging may be applied to early determination of treatment
efficacy. For example, molecular imaging using 18F-fluorothymidine (18F-FLT),
an indicator of the enzymatic activity of thymidine kinase 1 (TK1), which
highly correlates with cell growth potential, is considered to be effective
for early determination of the efficacy of an anticancer drug. We have
recently evaluated the effectiveness of molecular imaging using 3H-FLT
in the early determination of the efficacy of gefitinib in selectively
inhibiting phosphorylation in EGFR intracellular domains. The results indicated
that FLT rapidly reflects the efficacy of gefitinib and is effective for
the early determination of the efficacy of molecular targeted anticancer
drugs such as gefitinib (figure).
(Figure) Quantitative evaluation of FLT uptake in tumor tissue after treatment
of tumor-xenografted mice with gefitinib.
(2) Evaluation of efficacy of radiation therapy by molecular imaging for
assessing
tumor growth potential
Radiation therapy is considered an important cancer treatment strategy
similarly to surgery and chemotherapy. In radiation therapy, early and
accurate assessment of changes in the state of tumors, such as posttreatment
tumor growth potential, is essential for selecting an effective treatment
strategy with few side effects. For this purpose, positron emission tomography
(PET) using 18F-FLT is expected as a technique for noninvasively visualizing
tumor growth potential. Using mice transplanted with human head and neck
cancer cells, we have examined the effectiveness of 18F-FLT PET for assessing
the change in tumors after radiation therapy and their growth potential.
The results indicated that 18F-FLT PET can be used to predict the growth
of tumors after radiation therapy and is effective for the early determination
of the efficacy of radiation therapy and the development of an appropriate
cancer treatment plan (figure).
(Figure)
FLT autoradiography (ARG) and histopathological images of tumor in mice
transplanted with cancer cells
【 Papers 】
1. Zhao S, Kuge Y, Zhao Y, et al. BMC Cancer. 2013;13(1): 525
2. Murakami M, Zhao S, Zhao Y, et al. Oncology letters. 2013;6(3):667-672.
3. Fatema CN, Zhao S, Zhao Y. Ann Nucl Med. 2013;27(4):355-362.
4. Nagane M, Yasui H, Yamamori T, et al. Biochem Biophys Res Commun. 2013;437(3):420-425.
5. Hatano T, Zhao S, Zhao Y, et al. Int J Oncol. 2013;42(3): 823-830
【2】 Diagnostic imaging of atherosclerotic lesions
(vulnerable plaques)
(1)Exploration of biomarkers for diagnosis of progression of atherosclerotic
lesions
Recently, plaque rupture in arteries and the resulting thrombus formation have been found to be closely related to the development of myocardial and cerebral infarctions. Hence, accurate diagnosis of the progression of atherosclerotic lesions is essential. Various biological molecules are associated with the progression of atherosclerotic lesions and should be comprehensively analyzed. We carried out proteomic analysis of aortic tissues in a mouse model of atherosclerosis and successfully identified 29 proteins whose expression levels increased in the arterial tissues with the progression of atherosclerotic lesions. Currently, we are examining the changes in the expression levels and localizations of these proteins in atherosclerotic lesions by immunohistochemical staining. The changes in the levels of these proteins in blood are also examined by enzyme-linked immunosorbent assay (ELISA) to evaluate in detail their association with the progression of atherosclerotic lesions. The results of these examinations will be the basis for selecting biomarkers suitable for functional imaging diagnosis, such as nuclear medicine diagnosis, and biochemical tests.
【 An application for a patent】
1. Sakamoto T, Hanzawa H, Manri N, Kuge Y, Japan Patent 05066599 (17, August,
2012)
2. Hanzawa H, Sakamoto T, Manri N, Kuge Y, Japan Patent 05337096 (09, August,
2013)
3. Hanzawa H , Sakamoto T, Manri N, Kuge Y, Japan Patent 05379616 (04,
October, 2013)
(2)Evaluation of efficacy of angiotensin II receptor blocker (ARB) for
atherosclerosis
by molecular imaging
Inflammation and apoptosis are considered to be important factors related to the vulnerability of atherosclerotic lesions. We have demonstrated using a mouse model (apoE-/- mice) that 18F-FDG (14C-FDG, an indicator of the activity of inflammation) and 99mTc-annexin A5 (a marker of apoptosis) accumulated at high levels in atherosclerotic lesions. Recently, we have also demonstrated using the above-mentioned tracers that ARB reduced inflammation and apoptosis in ARB-treated apoE-/- mice (figure). Our experimental results indicated that minimally invasive nuclear medicine imaging is effective for clarifying the changes in the state of lesions by the treatment and determining the direction of the treatment.
Amelioration of inflammation and decrease of FDG accumulation in vulnerable plaque after ARB treatment.
Amelioration of apoptosis and decrease of 99mTc-Annexin accumulation
in vulnerable plaque after ARB treatment.
【 Papers 】
1. Zhao Y, Zhao S, Kuge Y, et al. J Nucl Med. 2013;54(8):1384-1388.
2. Zhao Y, Zhao S, Kuge Y, et al. Mol Imaging. 2013;12(5):300-309.
3. Yamashita A, Zhao Y, Zhao S, et al. Circ J. 2013;77(10);2626-2635.
【3】 Development of new imaging agent using thymidine phosphorylase (TP) as a target
TP is an enzyme expressed at high levels in tumors and has been found to be closely related to the malignancy of cancer because TP not only causes angiogenesis but also induces infiltration and metastasis of cancer cells. We are developing nuclear medicine imaging agents targeting TP for clinical applications. Thus far, we have succeeded in synthesizing a 123I-labeled TP inhibitor (TPI) with uracil as a parent skeleton and demonstrated in an animal experiment that the 123I-labeled compound specifically accumulated in tumors with a high TP expression (figure). With the development of these imaging agents, not only will early diagnosis of tumors become possible, but information useful for predicting treatment efficacy, which was difficult for conventional agents, will also be obtained. Currently, we are preparing for first-in-man (FIM) tests on the basis of the results of previous clinical tests.
【 Papers 】
1. Li H, Zhao S, Jin Y, et al.: Nucl Med Commun. 2011; 32: 1211-1215.
2. Akizawa H, Zhao S, Takahashi M, et al.: Nucl Med Biol. 2010; 37: 427-432.
3. Takahashi M, Seki K, Nishijima K, et al.: J Label Compd Radiopharm.
2008; 51: 384-387.
4. Takahashi M, Seki K, Nishijima K, et al.: Heterocycles. 2008; 76: 237-241.
Central Institute of Isotope Science
Hokkaido University
Nishi 7, Kita 15, Kita-ku, Sapporo, Hokkaido 060-0815
TEL:+81-11-706-6088
(Administrative office)
TEL:+81-11-706-6087
(Office room)
FAX:+81-11-706-7862